Guiding healthcare providers on sterilising techniques
Standards providing guidance in healthcare
Sterile products and sterilising methods are guided by Standards, setting the benchmark for their performance, testing and outcomes. Whether they are products everyone can use such as hand sanitisers to processes to clean reusable medical equipment, Standards guide us towards a safer environment that promotes health and wellbeing.
Without the right sterilising methods and products, healthcare providers and their patients could be at greater risk of spreading or catching infections. The use of reusable medical devices is essential for many tasks. The Australian adoption of the international Standard AS/NZS ISO 11137 series provides guidance on sterilising healthcare products. This series includes information for raditation requirements for development, validation and routine control of sterilisation processes, guidance on dosimetric aspects and establishing the sterilisation dose.
On top of equipment, the safe reprocessing, storage, handling and transportation of reusable medical equipment must be taken into consideration to ensure the products remain safe for use throughout all production and transport stages. AS/NZS 4187:2014 specifies the requirements and processes for effective practices throughout all stages of the medical device.
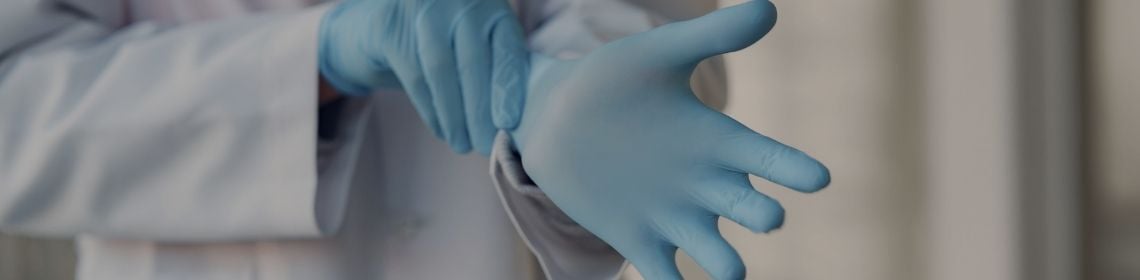
Single use items also have a place in a safe, sterile environment. Take rubber gloves for example, both the user and the patient require protection from cross-contamination throughout surgeries. AS/NZS 4179:2014 takes the ISO 10282:2014 Standard for single-use sterile rubber surgical gloves on board to adopt requirements for packaged sterile rubber gloves in Australia.
The development and implementation of healthcare Standards can drastically lower the chance of infections spreading, creating a safer environment for everyone.

Standards for Face Masks
Personal protective equipment (PPE) Standards
Standards play an important role in determining the effectiveness of personal protective equipment (PPE). PPE includes clothing and other equipment with the intention to protect someone from risks of injury or illness.

Understanding ISO 14971:2019
The medical device risk management Standard
The third edition of ISO 14971:2019 was published in December 2019 and provides a thorough process for manufacturers within the medical device industry to assess, monitor, and control risks in the creation and use of a medical device.

Quality Management Systems in Medical Devices
ISO 13485 specifies requirements for a comprehensive QMS
ISO 13485 is the internationally recognised Standard to assist those specifically in the Medical Devices industry to create a quality management system (QMS) that helps ensure safe and high-quality products are delivered.